A chain reaction to spare the air
Twelve billion tons of carbon dioxide spew into the air every year from power plants burning coal, oil and natural gas around the world. And energy demand only keeps growing.
The need to generate more electrical power can lead in two directions, and both exact huge costs. Power plants can continue to release high levels of the greenhouse gas, or they can adopt "carbon capture" technologies to trap and sequester some of the CO2 from emissions—but at great expense.
Hundreds—perhaps thousands—of university and commercial research centers test new materials and novel engineering schemes to more efficiently snare CO2 before it goes up the power plant flue.
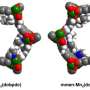
Last year, in the high-profile journal Nature, Jeffrey Long, professor of chemistry and of chemical and biomolecular engineering, reported devising a new material that can capture and release CO2 at a lower temperature and in a much greater volume than present-day technologies.
Built up to scale for use in a power plant, this would divert 95 percent of CO2 pollution from streaming out of power plant flues, and at the same time save as much as 50 percent on energy costs to do so.
As in the few power plants where carbon capture is currently being tested, carbon dioxide diverted from emissions would then be injected underground—sequestered—or stored and sold for industrial uses.
This year, with support from the Bakar Fellows Program, Jeffrey's lab has begun work to efficiently synthesize the new material at a large scale, to render it into a practical pellet form and confirm its greatly increased CO2 capture performance under realistic flue gas conditions.
"Unfortunately, we need to be doing CO2 capture now. It has become a really scary problem," Long says.
Current technologies capture CO2 in a liquid containing amines—organic molecules made up of nitrogen atoms that strongly attract the CO2 molecules. Long's team chose to mimic this basic structure, but in a solid powder form since it takes far less heat to release gases from a porous solid than a liquid.

They tweaked the composition to create a new molecular configuration that prompts a burst of CO2 uptake and release, accounting for the much greater yield. At certain temperatures, CO2 molecules fly off, one after the other, each molecule's departure triggering a chemical change in the structure that releases the next CO2 molecule in a chain reaction that Long calls cooperative adsorption.
The arrangement of the atoms in the material naturally forms a pore in the center. Adjacent CO2 molecules stick to the pore wall and link up to create
a continuous chain running along the edges of the tunnel-like pores. This enables the CO2 to make a quick exit when it is released—potentially into storage instead of up the flue.
The two unique traits capture and then release CO2 with significantly less heat.
"It's a long way from basic research to full commercial application," Long says. "Full-blown power plants are almost incomprehensibly big. But I would say that our material is the first example of this cooperative adsorption. No one had seen this before."
Long reported the preliminary results of the research in 2012, and two years later he launched a company, Mosaic Materials with two former graduate students to take the first steps along the long road to commercializing the technology, an effort now gaining momentum with support from the Bakar Fellows Program.
Producing the remarkable properties of the new white powdered material was really serendipitous, he says. "We wanted to make a solid adsorbent instead of the conventional liquid, since a solid can do the job with less heat input. We came up with the modifications of the typical composition, and it just happened that the spacing was right for this chain reaction effect."
Thomas McDonald, then a graduate student in Long's lab and now Mosaic Material's CEO, made the discovery and went on to figure out the chain reaction mechanism. Since developing the new material to full commercial scale is so demanding, the team is taking it one step at a time. The Navy supports some of Mosaic's current work. The military is looking for a better way to scrub out high levels of exhaled CO2 in submarines.
Devising a new material with properties even greater than expected is a thrill, Long says, but he values the real-world bonus.
"I'm interested in trying to do something with our science that could actually help the planet. It helps that this possibility really fires up some of the very bright students, who come to Berkeley to do Ph.D. research."
Journal information: Nature
Provided by University of California - Berkeley